Sanford surgeon’s groundbreaking device being tested at hospitals
This piece is presented by South Dakota Biotech.
Until now, patients with thoracoabdominal aortic aneurysms haven’t had many good options.
Left untreated, the condition – a balloon in a segment of the wall of the aorta – can rupture and cause sudden death.
And open surgery is far from simple.
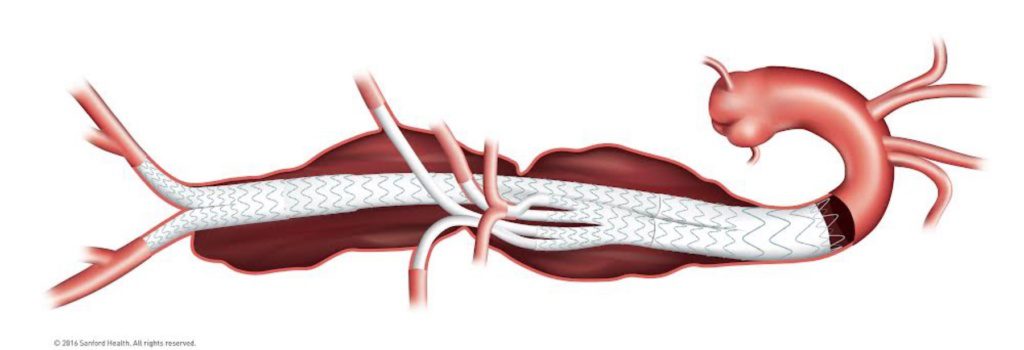
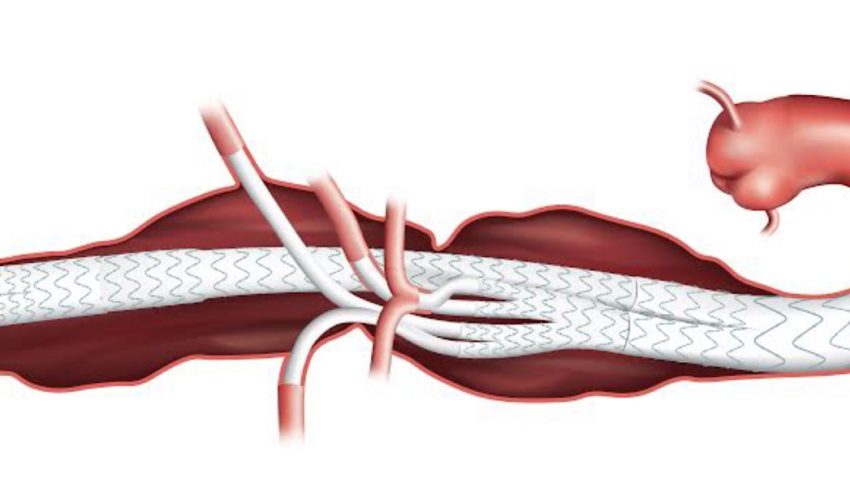
Thoracoabdominal aneurysms, which are especially rare, start in the thorax, extend through the abdomen and generally involve the branch arteries that supply blood to the liver, spleen, intestines, kidneys and other organs. Combined with their location in the aorta, these aneurysms are challenging to treat.
“It’s a very tough population to take care of,” said Dr. Pat Kelly, a vascular surgeon with Sanford Health. “There’s a very high morbidity with open surgery, and we’re hopeful through our device we can reduce that.”
Kelly invented his device – a stent graft system for a less-invasive treatment of these aneurysms – in Sioux Falls. As he describes it, “we basically reline the aorta to exclude their aneurysms. We fix their pipe inside their pipe. We fix their aneurysms from the inside.”
He began using it at Sanford in 2012 and signed a licensing deal with Medtronic in 2015 to manufacture the devices so other physicians could study them.
That’s now starting to happen.
“We have people knocking on the door to do this,” Kelly said. “We’re solving a problem nobody really has a solution for.”
One of the first surgeons asking to use the device was Dr. Geoffrey Answini, a thoracic and cardiac surgeon at The Christ Hospital in Cincinnat.
“When we were evaluating it, we were very impressed with what he was doing,” said Answini, who was part of a group of surgeons who visited Kelly and the engineers who worked on the device. “And after looking at this and what others had done around the country, we thought this was the best mousetrap to fix a difficult problem.”
After receiving access to the device this fall, Answini was able to treat a patient who had had multiple aneurysms and was unable to tolerate another open surgery.
“He’s been waiting two years. It’s not ideal. Some people can survive that long, and some don’t. You don’t know when these things are going to rupture,” Answini said.
“When we finally got it, he was so happy and relieved. The next day (after the procedure), I went into his room, and we literally hugged each other and gave each other a high five. The fact he’s able to even hug you after surgery shows how much easier it is to tolerate the operation. He would have been intubated on a ventilator before we even could have talked to him before.”
That procedure and a subsequent one both “went great,” Answini said.
“This technology is something we’re planning on using for a long time. We’ll be one of the only sites within a several-hundred-mile radius that has access to this, so we’ll see patients coming from multiple states.”
The device also is being tested at sites in New York and Tennessee, with others to come.
The fact that a medical device has gone from its first use to an industry study backed by a major player such as Medtronic in five years is impressive, said Joni Johnson, executive director of South Dakota Biotech.
“Most medical devices take a decade or more to see this kind of progress. I think it truly speaks to the groundbreaking work Dr. Kelly is doing,” she said. “Medical device development and manufacturing is an area of our biotech economy where we see a lot of potential to grow, and to have an example like this of what’s possible in South Dakota is very powerful.”
Following a industry-sponsored study, the hope is for final FDA approval that would allow the device to become available more broadly.
“I’m hoping within the next 18 months, but who knows,” Kelly said. “And they may look at it and say it doesn’t fit the plan and we’re going to shelve it. There’s always that possibility. It’s not done until it’s done. It’s a very humbling journey.”
Kelly is gracious to share access to his technology, Answini added.
“Some people when they invent something like this would keep it all to themselves, and he’s truly a humanitarian thinking about what’s best for the patient. He wants to get this technology out to help people,” the Ohio surgeon said.
“And it’s going to help people around the world. There’s no doubt about it. He’s onto something big here.”