Sanford teams work across spectrum to find answers to COVID-19
Sept. 16, 2020
This paid piece is sponsored by Sanford Health.
Whether patients have mild COVID-19 symptoms or are seriously ill, there’s a team at Sanford Research working to address and combat every step of the novel coronavirus. In its state-of-the-art research lab, the organization is leading the way with one of the nation’s most comprehensive approaches to research of the disease, including clinical studies of antibodies, treatments and more to bring discoveries at the bench to patients as quickly as possible.
“Never in the history of medicine have we been asked to define the natural history of a disease so quickly,” said David Pearce, president of Sanford Research, Innovations and World Clinic.
“With our research here and with our partners across the world, we have a much better understanding of what COVID is about now,” Pearce said. “We’ve been leading clinical trials, so if you’re hospitalized, we have options for you. If you’re intubated, we have options for you.”
COVID-19 clinical trials
Sanford Health is developing clinical trials to address every stage of the virus, a nod to its commitment to advancing the science of medicine and finding treatment options to defeat this deadly virus.
Listen now: David Pearce breaks down the four phases of a clinical trial
“Our physicians have done a phenomenal job in terms of understanding what the best care is for someone who has COVID right now,” Pearce said.
While some patients are more likely to face serious illness, exploring additional treatment options may be necessary, for example, for those with a weakened immune system, hypertension, cardiovascular or kidney disease.
“Our initial focus has been developing clinical trials that can aid and help people that may be more susceptible to attributes of the disease.”
Learn more: Find a Sanford Research clinical trial that’s right for you
SAB-185
Earlier this month, Sanford Health became the first in the nation to dose a patient with a promising novel therapeutic for COVID-19.
The new therapy, SAB-185, is a first-of-its-kind human polyclonal antibody therapeutic candidate developed by SAB Biotherapeutics, a biopharmaceutical company familiar to Sanford Health. SAB is housed at Sanford Research. The clinical trial will enroll patients across several clinical sites for a new therapy designed to treat people with mild-to-moderate COVID-19 at an early stage.
“This milestone underscores our relentless commitment to advancing the science of medicine to ensure our patients benefit from new discoveries as quickly as possible,” Pearce said. “Working with SAB Biotherapeutics on this clinical trial gives us an opportunity to deliver on our promise to patients.”
I-SPY
In August, Sanford introduced an option for the most critically ill patients battling complications from COVID-19. The I-SPY trial administers a medication, or combination of medications, for patients requiring a high flow of oxygen.
The goal is to rapidly identify therapies to treat critically ill COVID-19 patients.
“We are always pushing at Sanford Health to be on the front lines of emerging trends,” said Dr. Paul Berger, the lead physician for the clinical trial and a specialist in respiratory and critical care medicine. “To be able to offer this experimental therapy to the most ill patients while gathering data that can potentially help many more patients in the future is a major step forward against this new virus.”
Stem cell treatment
Sanford recently announced it has opened a Phase 1/2a trial using stem cells from umbilical cord linings, or ULSCs, to treat patients with moderate to severe cases of COVID-19. This also is the first of its kind in the U.S.
Stem cells from the umbilical cord are often discarded at birth, but they have a curative nature and anti-inflammatory component. These stem cells could help people who get really sick from COVID-19 by dialing down their immune response that overreacts to the virus and ends up attacking their own cells, causing inflammation.
The randomized, placebo-controlled and blinded study will look at whether infusing patients with ULSCs may be a safe and effective treatment for COVID-19.
“We are optimistic about the potential improvement with this treatment,” said Dr. W. Chad Spanos, principal investigator of the clinical trial at Sanford Health. “We look forward to enrolling more patients onto this trial and bringing promising new treatment options to our patients’ bedside in the future.”
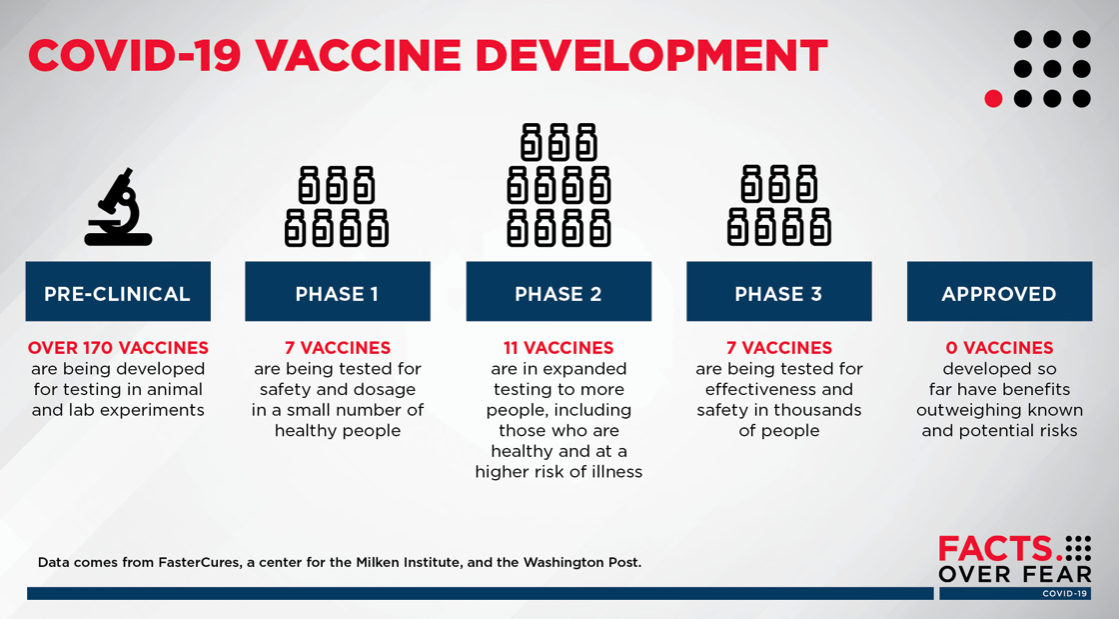
Developing a vaccine
The World Health Organization is tracking more than 170 vaccine candidates across the globe. At this point, no vaccine has reached FDA approval. Sanford Health will continue to collaborate with world-class partners to bring the best discoveries and treatments to patients first.
Collecting data to advance science through the pandemic
In January, Sanford Health proactively opened a COVID-19 registry to collect data from patients with the virus. To date, more than 13,000 patients are part of the registry. The data will allow Sanford’s researchers to track the progression of the disease and the recovery process for up to five years. It will aim to answer important questions around disease progression, the development of immunity, the possibility of reinfection and long-term health complications from COVID-19.
Pearce is thankful for Sanford’s dedicated investment in research.
“We’re coming through with that and saving lives every day as a team.”