Sanford makes big research announcements: Expanded stem cell trials, diabetes progress
April 27, 2018
The Food and Drug Administration recently approved two new clinical trials involving adipose-derived stem cells at Sanford Health, and significant progress is being made in research toward curing type 1 diabetes.
Those are among the headlines emerging this week at Unite to Cure: The Fourth International Vatican Conference, which brings together leaders in health care, science and research from around the world as part of the Cura Foundation conference, which is held every other year in Rome. This is the second time Sanford has presented at the invite-only event.
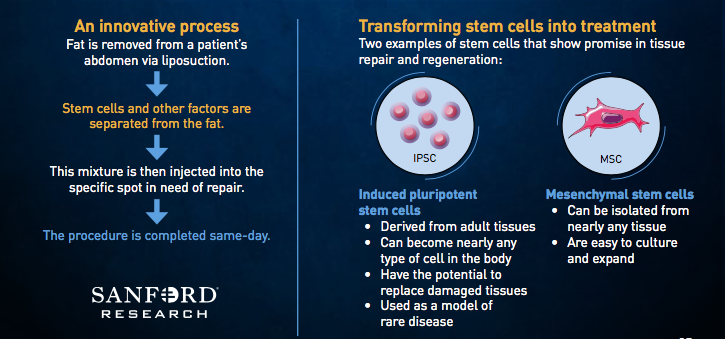
Sanford is sharing with the conference that it now has four FDA-approved clinical trials involving adipose- derived stem cells, with a fifth pending.
The health system had the first FDA-approved adipose-derived stem cell trial in the United States in 2016, with cells taken from a participant’s own abdominal fat used to treat rotator cuff injuries. That trial reached full enrollment in May 2017. The one-year follow-up visits are ongoing for enrolled patients and will be completed in May 2018. Data will be reviewed and study reports will be completed by the end of summer 2018.
“We know the future of medicine is trying to help the body repair itself, and we’re doing everything we can to move in that direction,” said David Pearce, vice president of research and innovation for Sanford Health. “Having FDA approval for these studies – and having so many of them – validates the work we’re doing.”
The latest trials are:
- The rotator cuff pivotal study opens in July. It will be a continuation of the initial rotator cuff study and further investigate the potential effects of adipose-derived stem cells on rotator cuff tears. It will enroll 210 participants nationwide, with 30 from Sanford Health locations in Sioux Falls and Fargo. Participants will receive an injection of stem cells or standard of care treatment.
- The facet joint study is a feasibility trial that opens this summer. The trial will investigate the potential healing factors of adipose-derived stem cells in patients with osteoarthritis of the facet joints. Patients must have chronic lumbar back pain caused by facet joint osteoarthritis for more than six months and have failed three months of conservative back pain care. This study will enroll 40 patients — 20 will undergo a liposuction procedure, and 20 will receive standard of care treatment.
- The wrist osteoarthritis study is a feasibility trial that opens in June. It will investigate the potential healing factors of adipose-derived stem cells in patients diagnosed with wrist osteoarthritis. There will be 40 patients enrolled in this study. All patients will receive a minor liposuction procedure, and then half will receive stem-cell injections, and half will receive a steroid shot. Participants will not know which treatment they receive.
A trial for non-healing leg wounds that opened in September 2017 continues. The feasibility trial will study the safety and efficacy of using adipose-derived stem cell therapy as a treatment for the wounds. The trial will accept 36 participants.
Cell therapy, according to Sanford Health experts, uses the body’s own cells as therapy. Stem cells, in particular, have the ability to repair or regenerate cells that are damaged or killed as the result of injury or disease. Sanford Health’s cell therapy techniques focus on adipose-derived stem cells because they can be used in many parts of the body and are easily collected. Adipose stem cells yield many times more cells than other sources like bone marrow. They also can be returned to the body quickly, and they have a low infection rate.
Sanford also is sharing its progress in trying to cure type 1 diabetes.
“New ways of monitoring blood sugar and dosing insulin are coming,” said Sanford’s Dr. Kurt Griffin.“But until we figure out a way to keep the body from attacking itself, we are limited to replacing insulin the body can no longer make. This is lifesaving, but it requires constant vigilance, and there is no way to take a break from therapy. That can be very frustrating.”
A desire to get to the root of the disease helps drive Griffin’s research, including the ongoing T-rex clinical trial.
This Phase 2 clinical trial is conducted collaboratively by Sanford Health and Caladrius Biosciences Inc.
The study opened in March 2016, and the first enrollee recently received his final evaluation as part of the study.
“This is a significant milestone, but it’s only the beginning of getting these children through the trial successfully,” said Griffin, lead investigator for the trial.
The trial has completed enrollment of 110 children with type 1 diabetes. The study started with sites at Sanford Health in Sioux Falls and Fargo and expanded to 13 additional sites across the United States.
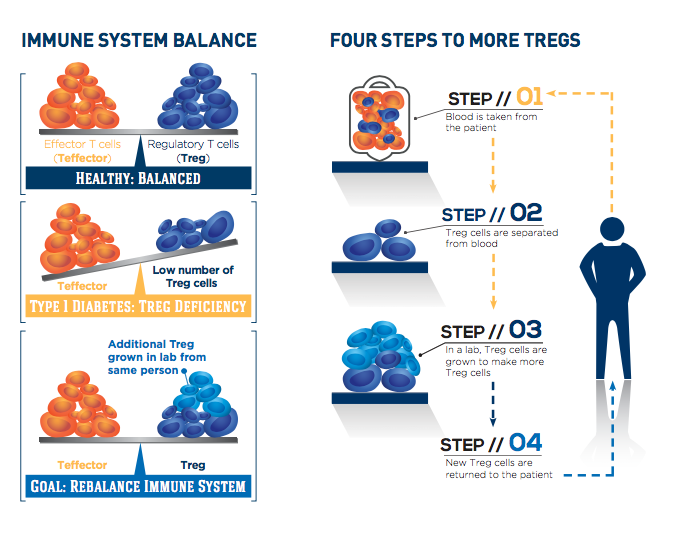
The T-rex trial is testing the potential of CLBS03, Caladrius’ cell therapy consisting of each patient’s own regulatory T cells, or Tregs, to help the body fight type 1 diabetes. After a single dose of expanded autologous Treg cells or placebo, subjects are followed for two years, with the primary endpoint of persistence of insulin production at one year after treatment.
People with type 1 diabetes experience a loss of insulin-producing beta cells as their immune system targets these cells inappropriately. Treg cells usually keep the immune system under control, but they are lacking in number and activity in people with type 1 diabetes.
The study is exploring whether expanding the body’s supply of Treg cells can rebalance the immune system, stop destruction of beta cells and preserve insulin production. Participants were randomized to either of two doses in the treatment groups or to placebo. For those in the treatment groups, the participant’s own Treg cells were extracted from the body, purified, expanded in culture and then returned to blood circulation. The cell identification and expansion process is patented technology licensed by Caladrius.
The therapy being used in this trial, CLBS03, has received fast-track designation from the FDA, a first for any type 1 diabetes intervention. That designation is reserved for drugs or biologics that address a serious health condition where there is an unmet medical need.
This status allows for more frequent communication with the FDA and faster feedback about the therapy during the approval process, Sanford said. It also allows researchers to submit data and reports on a rolling basis. CLBS03 also has been granted European Medicines Agency’s advanced therapeutic medicinal product classification and FDA’s orphan drug designation as a potential new treatment for recent-onset type 1 diabetes.
The conference has been a key way to share information and collaborate worldwide, Pearce said.
“It’s invaluable for us as researchers to be part of this conference,” he said. “These are some of the best and brightest in the industry, and every time we go, we are inspired by our colleagues to work harder to advance treatments and search for cures.”
Sanford to detail research, philanthropy at international conference