FDA names Sanford Health stent graft as ‘breakthrough’ device
Oct. 21, 2021
This paid piece is sponsored by Sanford Health.
An investigational device invented at Sanford Health that helps high-risk vascular disease patients has been granted a breakthrough device designation by the U.S. Food and Drug Administration because it fills an unmet need.
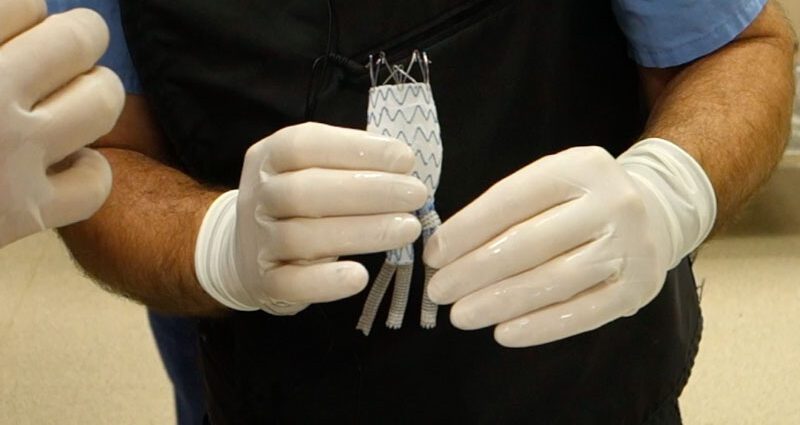
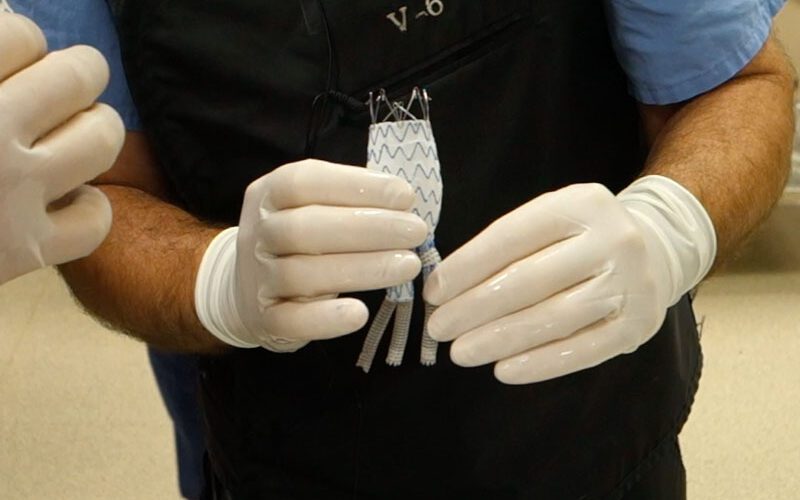
Dr. Patrick Kelly, a Sanford Health vascular surgeon, invented the aortic stent graft system designed to treat a thoracoabdominal aortic aneurysm, or TAAA. That is a complex condition that causes a dangerous bulging of the aorta extending from the chest down into the abdomen. It typically involves the branch arteries that supply blood to multiple internal organs. Left untreated, the aneurysm can rupture and cause sudden death.
The standard of care is complex open surgery, which is associated with a high rate of complications and mortality, and 40 percent of patients are not considered candidates for open surgical repair.
Kelly’s concept has the potential to treat more of those people utilizing his minimally invasive approach. Under a physician-sponsored investigational device exemption, or PS-IDE, he has treated more than 150 patients at Sanford Health over the past nine years who otherwise had no other options.
“This helps move the device through the regulatory pathway. It opens the door for more patients to have an option for repair, even the potential for patients with aortic dissections, failed prior repairs and anatomies that were previously thought to be untreatable,” Kelly said. “The non-anatomical design allows broad applicability of our patient population.”
That unmet need, leaving some patients with no other option, was key to the breakthrough device designation, said Katie Pohlson, senior director of innovation and commercialization at Sanford Health.
“With it, we will receive prioritized reviews, access to senior staff at the FDA and collaboration with the FDA to help with the development of the product,” she said.
“The significance for Sanford Health is our expertise in helping de-risk these technologies and bringing them forward to help benefit the public. We’re taking good care of our patients, but we’re also developing technologies that help people being treated at other health care organizations,” Pohlson said.